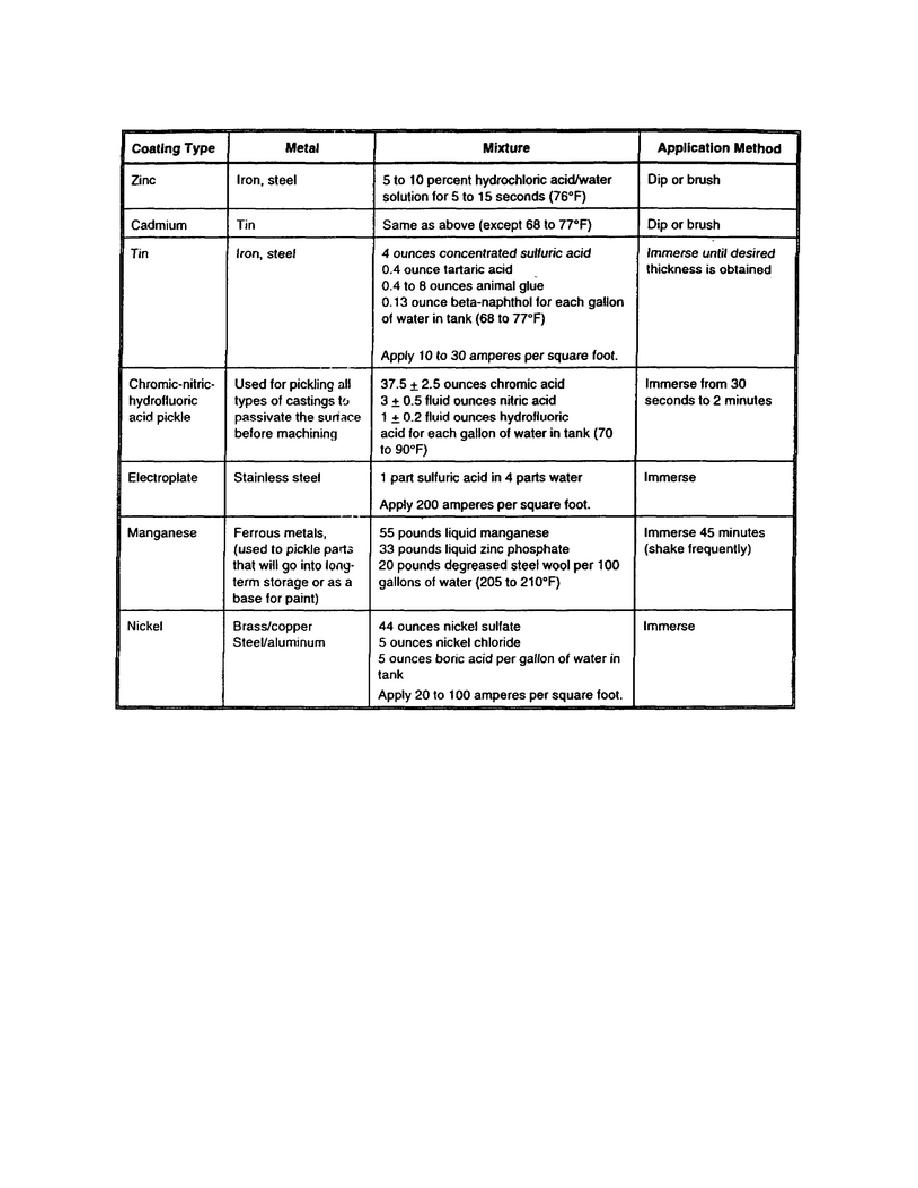
Table 5-3. Mixtures for passivating metal surfaces
(3) A metal's passivity is attributed, directly or indirectly, to a protective film that changes
the metal's or alloy's galvanic potential in a more noble direction. For example, the inhibiting action of
the chromate ion in zinc chromate priming paint provides protection by passivation or mechanical
means. The zinc chromate diffused in the paint provides a concentration of chromate ions on the metal's
surface and tends to passivate the base metal. The chromate film shares electrons from surface iron
atoms to make the metal surface less reactive and more noble in the galvanic series (Table 5-2, page 5-
7). Sodium nitrate is also used as a passivator and will render iron several tenths of a volt more noble
than iron in distilled water. The nitrate ion is oxidizing in nature and, like chromates and other oxidizing
passivators, reduces corrosion.
(4) The use of metallic coatings and claddings is another way of making a metal passive to
its environment. This means of metal protecting plates a metal's surface with another corrosion-resistant
metal. In some cases, plating gives the base metal a hard, wear-resistant surface in addition to providing
protection against corrosion.
5-15
EN0562



 Previous Page
Previous Page
