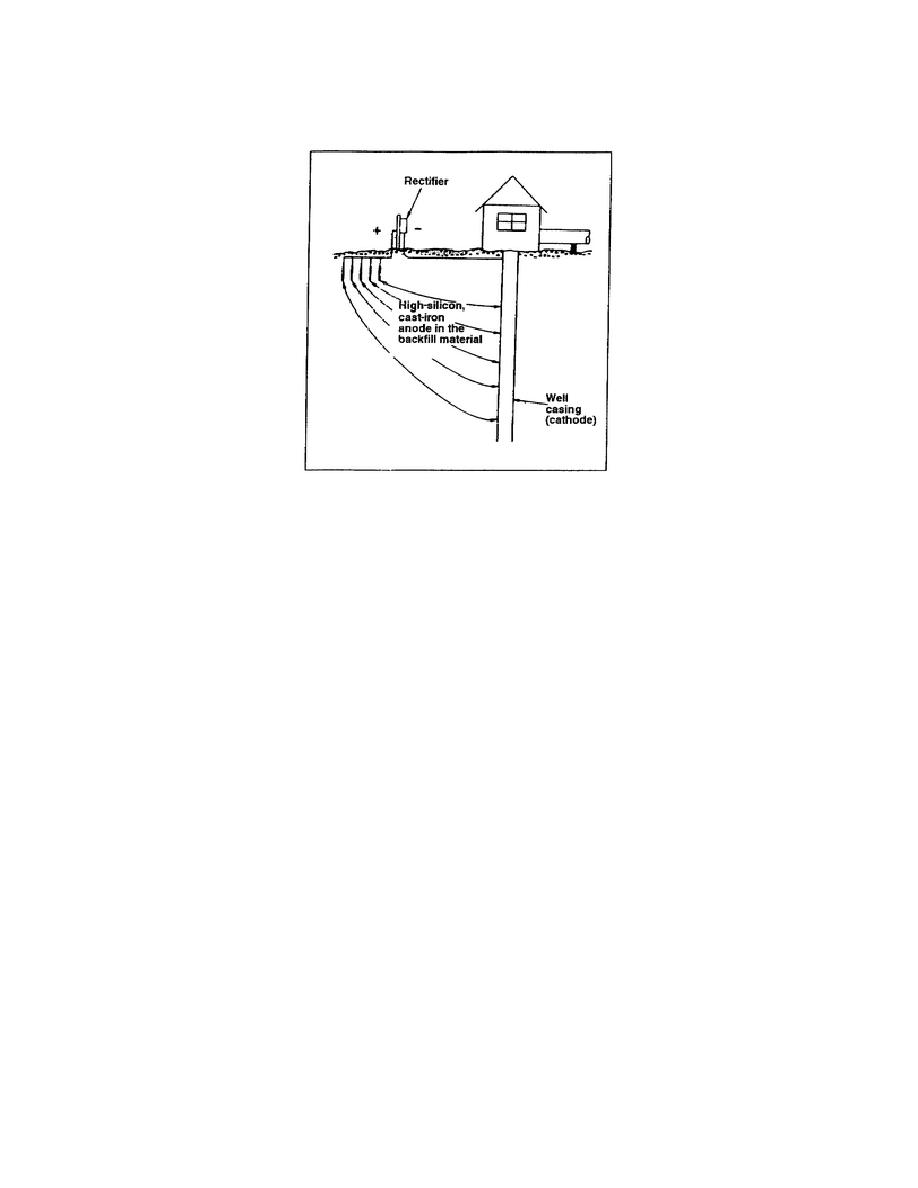
it, and prevents corrosion of the structure. Figure 5-11 shows a setup of an impressed current method of
cathodic protection.
Figure 5-11. Impressed current method of cathodic protection
PART B: METAL IDENTIFICATION
5-5. Metal-Identification Tests. Unknowingly, you identify metal everyday; for instance, a penny is
made of copper, dimes and quarters are made basically of silver, and nickels are made of nickel. You
can identify them by looking at them. Some metals are identified by their use, such as aluminum for
aircraft skin, and copper for electrical wire. However, this general classification is not good enough for
the purpose of corrosion control, because if you misidentify a metal type, the work you do may damage
the metal or create a condition that will increase the possibility of corrosion. By misidentifying metal,
you may waste your efforts, damage material, or cause corrosion. The first and most important step in
fighting corrosion is identifying the material that is being attacked or will require protection from attack.
There are other tests besides general classification that will help you determine what type of metal you
are working with, such as visual examinations and mechanical, chemical, and complex tests.
a. Visual Examination. This examination reveals numbers or color codes that will identify a
metal. The Society of Automotive Engineers (SAE) and the American Iron and Steel Institute (AISI)
have established a means of identifying steels with numbers and colors.
(1) Number system. The number system, which is a steel identification numerical index
(Table 5-4), works in the following manner. A four- or five-digit number is used to indicate the steel
type. For example, 2330 is a number code identifying a certain
EN0562
5-18



 Previous Page
Previous Page
