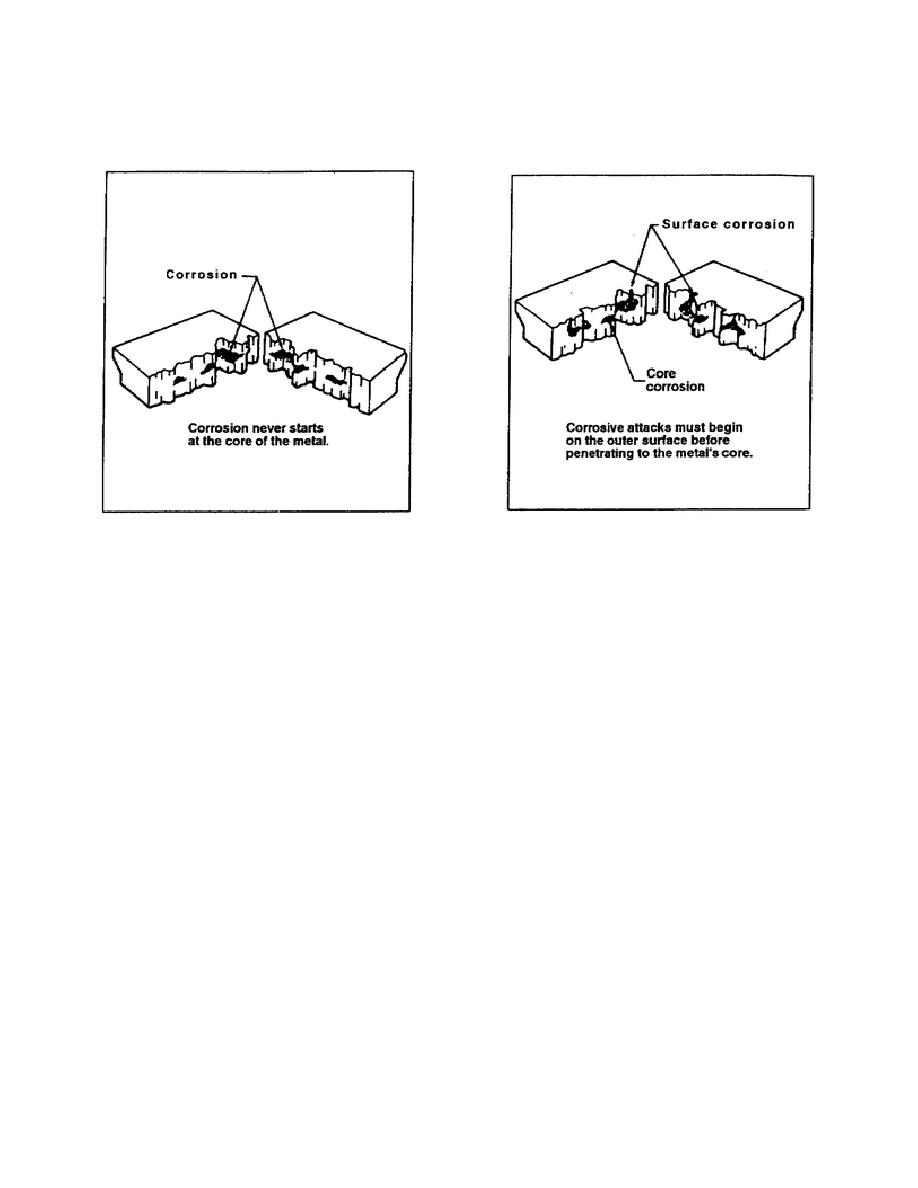
allowed to progress, corrosion works down into the core of the metal's material (Figure 5-4). Since
corrosion never originates in the core, there will always be evidence on the surface when an attack is in
progress (Figure 5-5).
Figure 5-5. Corrosion point of entry
Figure 5-4. Core corrosion
5-2. Forms. Before you can identify corrosion, you must know what it looks like on a metal's
surface. Corrosion takes place in various forms, depending on the metal type, the environment, and the
mechanical conditions. Among the many types of corrosion are the following:
a. Uniform (General). This corrosion is the most common form, which is a general attack on a
metallic surface. Uniform metal etching is the surface effect produced by most direct chemical attacks.
On a polished surface, this corrosion type is first seen as a dulled surface. If such corrosion is allowed to
continue, the surface becomes rough and possibly frosted in appearance.
b. Galvanic.
(1) This corrosion type is a complete corrosion class that involves an electrochemical action
between two metals or between different areas of the same metal having different heat treatments or
other metallurgical differences. Galvanic corrosion occurs when dissimilar metals are in contact and the
presence of moisture provides an external circuit (electrolyte). One recognizable feature of galvanic
corrosion is the presence of corrosion buildup at a joint between metals. For example, aluminum and
magnesium skins that are riveted together in an aircraft wing form a galvanic couple if moisture and
contamination are present.
(2) When aluminum pieces are attached with steel fasteners or screws, galvanic corrosion
(Figure 5-6) can occur between the aluminum and steel. Table 5-1, page 5-6, is a
EN0562
5-4



 Previous Page
Previous Page
