The Webster's Collegiate Dictionary, 10th ed, 1995, states, "The action, process, or effect of
corroding." Webster further defines corroding as, "To eat away by degrees as if by gnawing."
Though the definitions vary, they all boil down to the fact that corrosion is a natural act of a metal trying
to return to its lowest level of energy. In the case of a metal structure, iron and steel try to return to their
natural state of iron oxide (iron ore). The so-called noble metals, such as gold and platinum, do not
corrode since they are chemically uncombined in their natural state. Your concern is with the chemical
changes that occur when metal is exposed to the elements.
a. Electrochemical Theory. The most common theory of corrosion is called electrochemical.
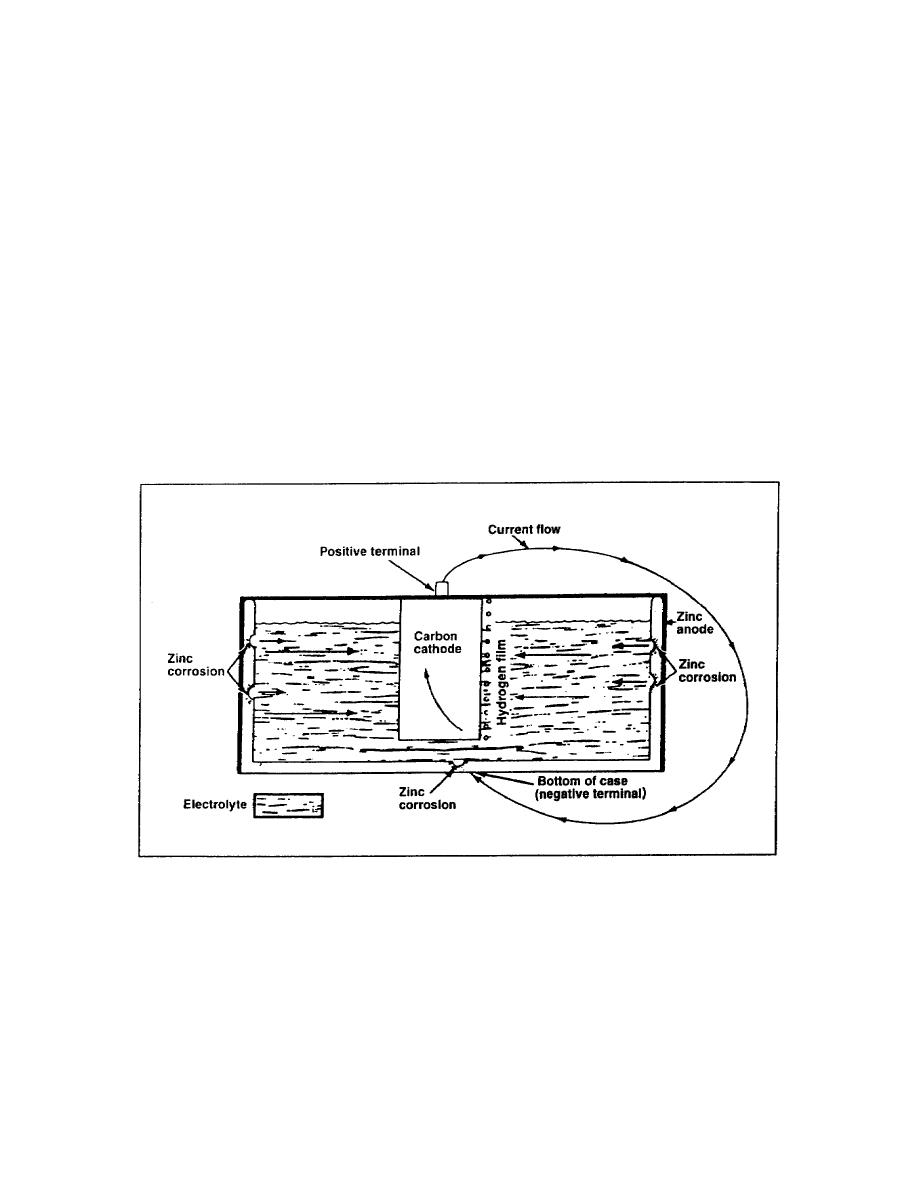
An electrochemical-corrosion theory is best explained by the action that takes place in a battery cell
(Figure 5-1). A galvanic battery cell is produced by placing two dissimilar metals in a suitable
electrolyte that is a conducting medium in which the flow of current is accomplished by the movement
of matter in the form of ions. The resulting electrochemical reaction develops a potential difference
between these two metals, causing one metal to be anodic and the other metal to be cathodic. In a dry
cell, the zinc case is the anode (positive terminal) and the carbon rod is the cathode (negative terminal).
When an external electrical circuit is completed, current flows from the zinc case into the electrolyte,
taking with it particles of zinc. This is an example of galvanic corrosion of the zinc case.
Figure 5-1. Electrochemical-corrosion theory of a battery cell
b. Electrochemical Conditions.
(1) Four conditions must exist before electrochemical corrosion can take place; namely, the
presence of a metal anode, a cathode, an electrolyte, and a conductor.
EN0562
5-2



 Previous Page
Previous Page
