_________________________________________________________________ Semiconductor Diodes
and so on, in accordance with the exclusion principle. Each of these shells is a major shell
and can be divided into subshells, of which there are four, labeled "s", "p", "d", and "f".
Like the major shells, the subshells are also limited as to the number of electrons that they
contain. So, the "s" subshell is complete when it contains two electrons, the "p" subshell
when it contains six, the "d" subshell when it contains ten, and the "f" subshell when it
contains fourteen electrons.
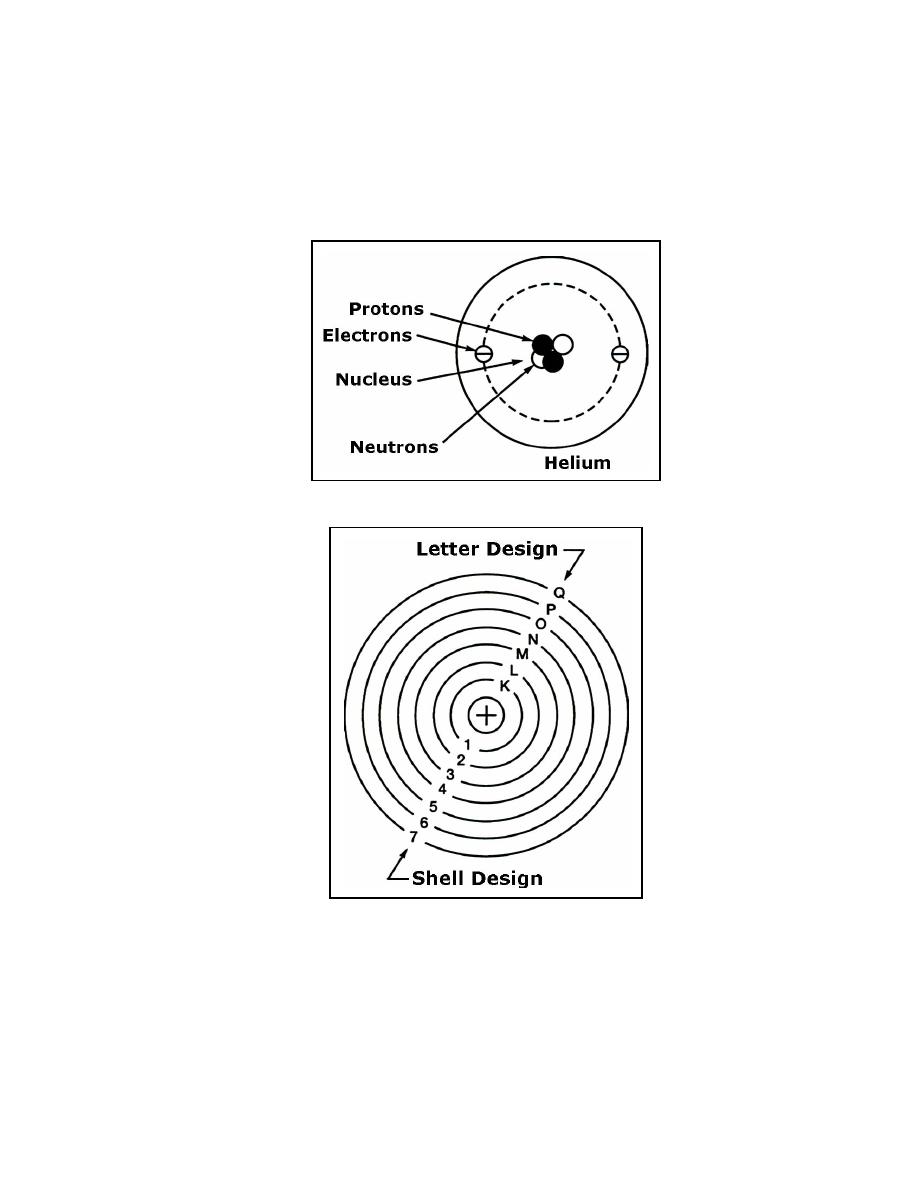
Figure 1-2. Composition of a Simple Helium Atom
Figure 1-3. Shell Designation
1-32. Since the "K" shell can contain no more than two electrons, it must have only one
subshell, the "s" subshell. The "M" shell is composed of three subshells: "s", "p", and "d".
If the electrons in the "s", "p", and "d" subshells were added together, their total would be
18, the exact number required to fill the "M" shell. Figure 1-4 shows the electron
configuration for copper. The copper atom contains 29 electrons, which completely fills
the first three shells and subshells, leaving one electron in the "s" subshell of the "N" shell.
23 June 2005
TC 9-62
1-7



 Previous Page
Previous Page
