TC 9-62
A list of all the other known elements, with the number of electrons in each atom, is shown
in Appendix B (Periodic Table of Elements).
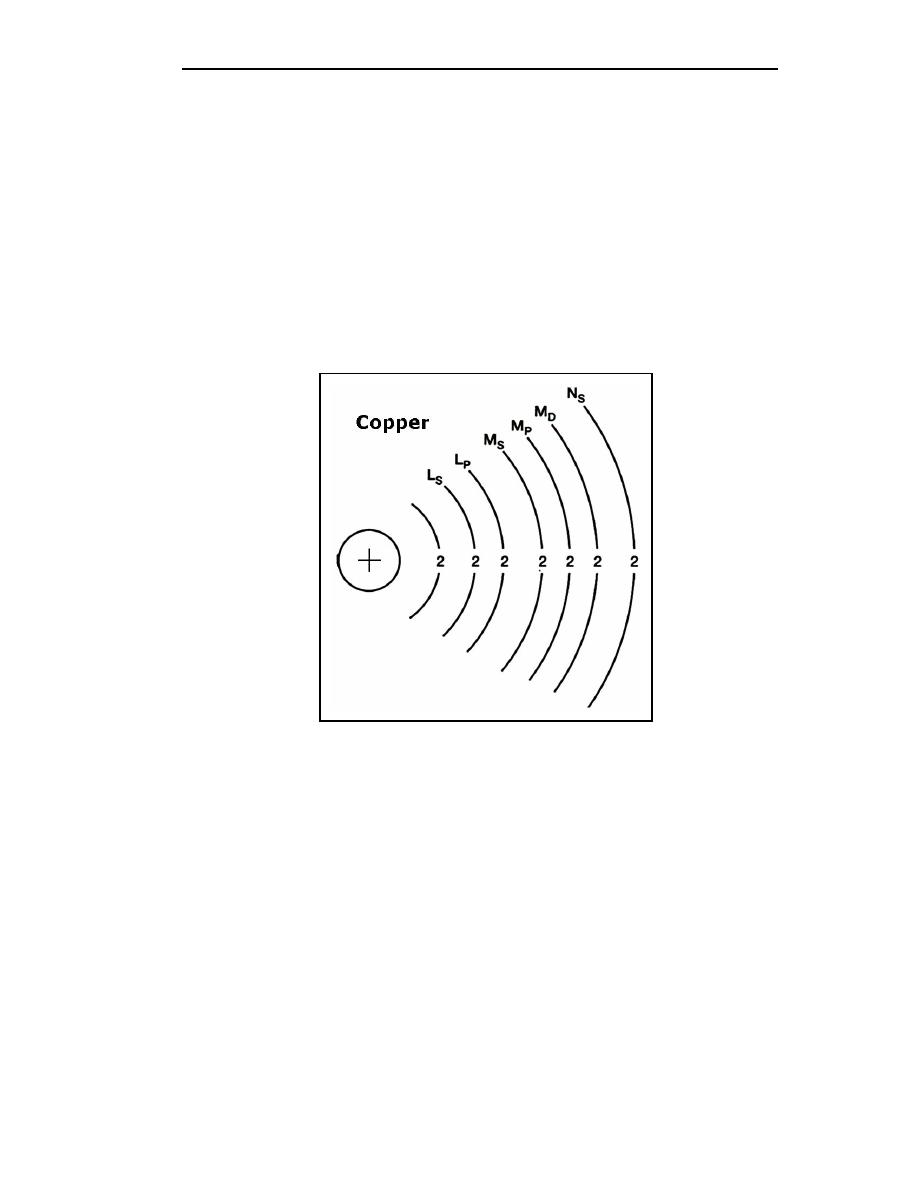
1-33. Valence is an atom's ability to combine with other atoms. The number of electrons
in the outermost shell of an atom determines its valence. Therefore, the outer shell of an
atom is called the VALENCE SHELL and the electrons contained in this shell are called
VALENCE ELECTRONS. The valence of an atom determines its ability to gain or lose an
electron, which in turn determines the chemical and electrical properties of the atom. An
atom that is lacking only one or two electrons from its outer shell will easily gain electrons
to complete its shell. However, a large amount of energy is required to free any of its
electrons. An atom having a relatively small number of electrons in its outer shell in
comparison to the number of electrons required to fill the shell will easily lose these
valence electrons. The valence shell always refers to the outermost shell.
Figure 1-4. Copper Atom
ENERGY BANDS
1-34. Remember, orbiting electrons contain energy and are confined to definite energy
levels. The various shells in an atom represent these energy levels. Therefore, in order to
move an electron from a lower shell to a higher shell a certain amount of energy is
required. This energy can be in the form of electric fields, heat, light, and even
bombardment by other particles. Failure to provide enough energy to the electron, even if
the energy supplied is just short of the required amount, will cause it to remain at its
present energy level. Supplying more energy than is needed will only cause the electron to
move to the next higher shell and the remaining energy will be wasted. In simpler terms,
energy is required in definite units to move electrons from one shell to the next higher
shell. These units are called QUANTA (for example, 1, 2, or 3 quanta).
1-35. Electrons can also lose energy as well as receive it. When an electron loses energy,
it moves to a lower shell. The lost energy, in some cases, appears as heat.
1-8
TC 9-62
23 June 2005



 Previous Page
Previous Page
